Most Atomic Solids Have Low Melting Points
In these solids the geometry is such that one side has a negative charge and the other has a positive charge. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structureThe chemical symbol for Lithium is Li.
15 Metals With The Lowest Melting Point Materials Science Engineering
Like all alkali metals lithium is highly reactive and flammable and is stored in mineral.
. Mercury is a liquid at room temperature and the alkali metals melt below 200 C. Under standard conditions it is the lightest metal and the lightest solid element. Covalent atomic solids non bonding atomic solids and metallic atomic solids.
Metals have high melting point while non-metals have low melting pointVerifiedHint. Examples of molecular solids with low melting and boiling temperatures include argon water naphthalene nicotine and caffeine see table below. These differences reflect differences in strengths of metallic bonding among the metals.
Excerpted from The Complete Idiots Guide to Chemistry 2003. Solids who composite units are individual atoms. That depends on the solid.
1Circle the letter of each sentence that is true about Daltons Atomic Theory aAll elements are composed of. 122 cubic closest packing. As a result of this unusual bonding amorphous solids have a very wide range of properties.
Moderately low Melting points. Tungsten has got the greatest melting point and for that reason exists as with solid condition at 70 degrees. Metals have high melting point while non-metals have high melting pointD.
Answered over 90d ago. Have high melting pointC. Nonbonding atomic solids have low melting points.
Atomic solids generally have low melting points Ionic solids tend to have higher melting points than molecular solids Based on your understanding of bonding in liquids and solids arrange the following substances from the highest to. What are ionic solids. An atomic solid held together by dispersion forces.
Some amorphous solids such as window glass are hard brittle and have a high melting point while other amorphous solids such as rubber or plastic are soft and have very low melting points. What are the three categories we can divide solids into. Molecular solids have low melting Tm and boiling Tb points compared to metal iron ionic sodium chloride and covalent solids diamond.
Examples of polar molecules are ethanol and ammonia. Ionic solids tend to have. 119 rows Periodic Table of Elements with Melting Point Trends.
The force holding them together is a dipole dipole force of attraction. A low melting point also means that the inter -molecular forces that cause attractions between one molecule and another are not very strong. Ice has a very low melting point lard and butter have low melting points chocolate has a relatively low melting point wax has an intermediate melting point lead has a.
It is a soft silvery-white alkali metal. Crystalline solid crystal Crystalline solids are orderly geometric structures in which atoms molecules or ions are arranged in patterns with long-range repeating order. Their melting and boiling points are higher than non-polar molecular solids but still relatively low.
For example a gas will have weaker intermolecular attractions than its liquid counterpart. The melting points of the metals vary widely. What are atomic solids.
Iron III chloride FeCl3 is an orange to brown-black solid at room temperature. It is used in drinking water productio. Much higher melting points than molecular solids.
Several post-transition metals also have low melting points whereas the transition metals melt at temperatures above 1000 C. Answered over 90d ago. A covalent bond is formed when two atomic orbitals have optimal orbital overlap and electrons are.
The combination of a relatively large atomic size and only one delocalized electron in the conduction band leads to a relatively low lattice energy and so a low melting point for all of the alkali metals and a melting point that predictably drops as you go down the group starting from lithium which melts at about 180 C until you reach cesium which melts at a little less than 30 C.

Pin By Sanghita Dey On Cbse Class 10 Ionic Compound Reducing Agent Ionic
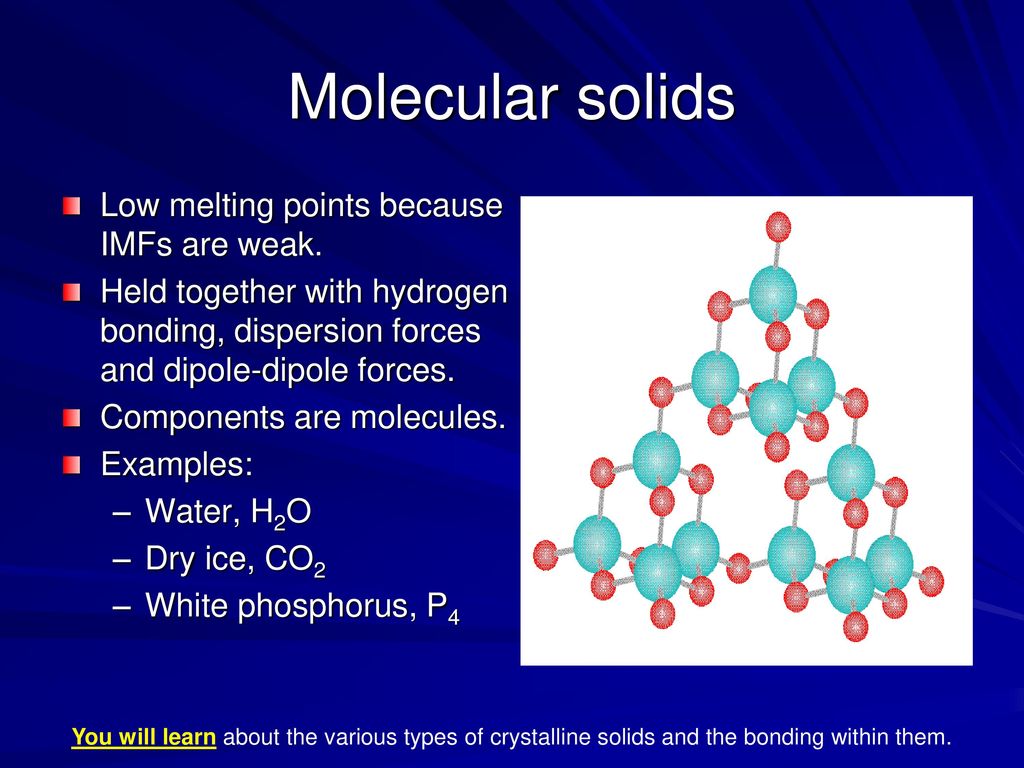
Chapter 14 Liquids And Solids Ppt Download

Crystalline Solids Band Theory And Doping Ppt Video Online Download

Focus 3 G J Liquids Solids Ppt Download
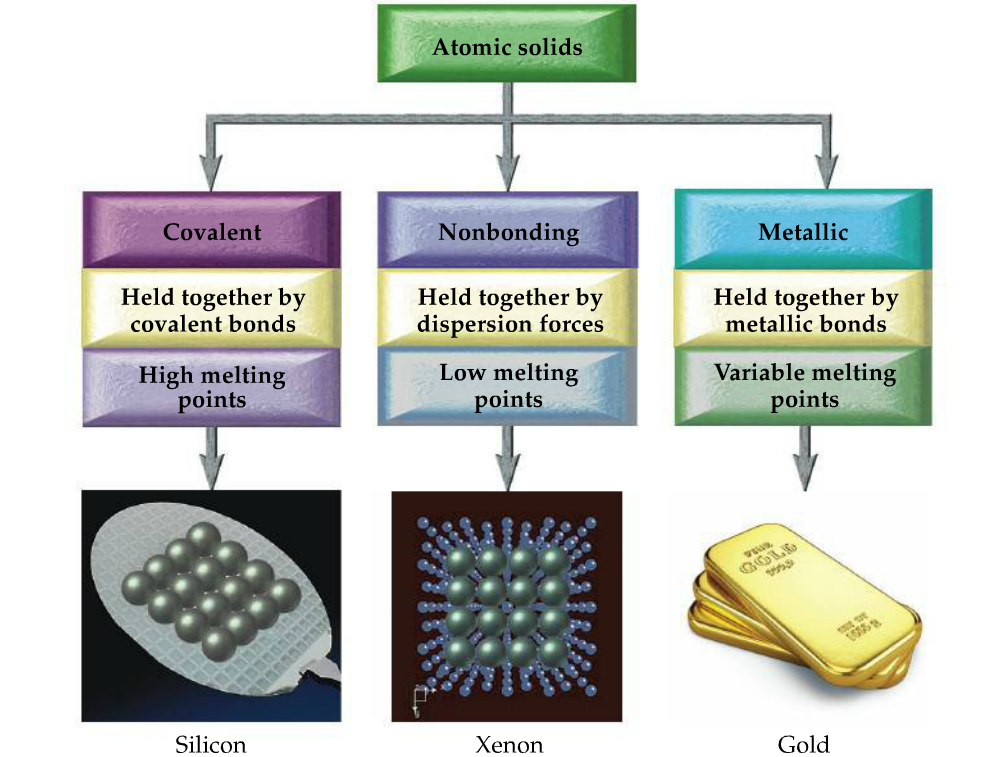
Solved 3 Point Suppose A Sample Of Water Is Contaminated Chegg Com

Question Video Comparing Molecular Solids And Covalent Networks Nagwa

Intermolecular Forces Ppt Download

Behavior Of Gases Low Density Compression And Expansion Ppt Download
15 Metals With The Lowest Melting Point Materials Science Engineering
15 Metals With The Lowest Melting Point Materials Science Engineering

Ionic Solids Molecular Solids Metallic Solids Network Covalent Solids Atomic Solids Youtube
Why Do Noble Gases Have Very Low Boiling And Melting Points Quora
Why Do Hydrogen Bonded Molecular Solids Have Low Melting Point Quora
15 Metals With The Lowest Melting Point Materials Science Engineering
![]()
Solids Chem Honors Ppt Video Online Download

Easy Chemistry With Unisprint 100 Steps To Sat Ii Chemistry Step 11 Properties Of Interatomic Bonds Ionic Teaching Chemistry Chemistry Chemistry Lessons

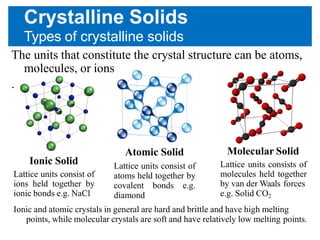

Comments
Post a Comment